Applications of First Law of Thermodynamics
Applications of First Law of Thermodynamics: Overview
This topic covers concepts, such as, Work Done in Reversible Adiabatic Process, Work Done in An Isothermal Expansion For Ideal System, Interpretation of Work from Graph of Cyclic Processes & Reversible Polytropic Process etc.
Important Questions on Applications of First Law of Thermodynamics
During isothermal expansion of an ideal gas, its
Absolute zero is defined as the temperature
moles of an ideal gas expands isothermally against a constant pressure of Pascal from L.The amount of work involved is
One mole of an ideal gas is taken from and along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is and that along the dotted line path is then the integer closest to the ratio is:
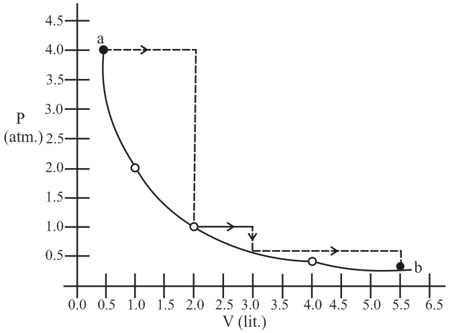
Two moles of gas are initially at temp and occupy a volume of litres. The gas is first expanded at constant pressure until its volume is doubled. Then it undergoes reversible adiabatic change, until the volume become , then predict the value of (where is the final temperature, )
The magnitude of reversible work done by an ideal gas in four different processes: isothermal expansion, adiabatic expansion, constant pressure expansion, and free expansion are , and respectively. Choose the right order of sequence for the magnitude of the work done. (Change in the volume is same for all the processes.)
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
and for the following process for a monoatomic gas are:
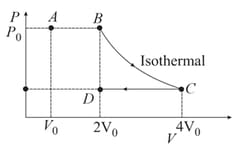
Consider the reversible isothermal expansion of an ideal gas in a closed system at two different temperatures and The correct graphical depiction of the dependence of work done on the final volume
A thermodynamic cycle in the pressure -volume plane is given below:
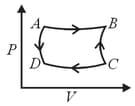
and are isothermal processes while and are adiabatic processes. The same cycle in the temperature - entropy plane is :
During the free expansion of an ideal gas in an isolated chamber,
One mole of an ideal gas (at ) is expanded from volume to at constant temperature. The value of work done (in ), if gas is expanded against vacuum ?
For next two question please follow the same
A sample of an ideal gas is expanded to twice its original volume of 1 m3 in a reversible process for which and at constant volume.
The work done during the process may be
moles of an ideal gas with at held in cylinder fitted with piston is expanded reversibly and isothermally to three times volume (initially ). The cylinder placed in an insulated container and pressure returned to adiabatically in one step when mechanical equilibrium is reached. Read the passage and answer the questions as single choice correct.
The volume at point may be
For next two question please follow the same
moles of an ideal gas with at , held in cylinder fitted with piston is expanded reversibly and isothermally to three times volume (initially ). The cylinder placed in an insulated container and pressure returned to adiabatically in one step when mechanical equilibrium is reached. Read the passage and answer the questions as single choice correct.
process may be
Among the following, which expression is most accurate to calculate the work done by gas in reversible isothermal expansion?
Adjacent figure shows the force-displacement graph of a moving body, the work done in displacing body from to by the shown force is equals to
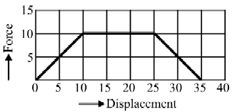
Graphically show the total work done in an expansion when the state of an ideal gas is changed reversibly and isothermally from to . With the help of a plot compare the work done in the above case with that carried out against a constant external pressure
An ideal gas is taken around the cycle ABCA as:
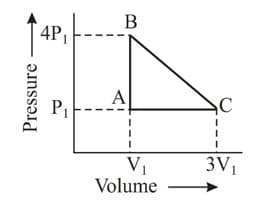
Calculate work done in the cyclic process?
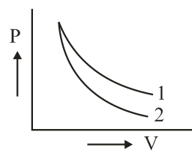
P-V plots for two gases during adiabatic expansion are shown in figure. Plots 1 and plots 2 should correspond respectively to